-
United States -
United Kingdom -
India -
France -
Deutschland -
Italia -
日本 -
대한민국 -
中国 -
台灣
-
Ansysは、シミュレーションエンジニアリングソフトウェアを学生に無償で提供することで、未来を拓く学生たちの助けとなることを目指しています。
-
Ansysは、シミュレーションエンジニアリングソフトウェアを学生に無償で提供することで、未来を拓く学生たちの助けとなることを目指しています。
-
Ansysは、シミュレーションエンジニアリングソフトウェアを学生に無償で提供することで、未来を拓く学生たちの助けとなることを目指しています。
ANSYS BLOG
January 5, 2023
How Regeneron Pharmaceuticals Transforms Products and Processes with Simulation
“You can’t drop what doesn’t exist,” says Ross Kenyon, Associate Director and leader of the Modeling, Simulation, and Analysis team at Regeneron Pharmaceuticals. But you can simulate it.
Regeneron is a leading biotechnology company that invents, develops, and commercializes life-transforming medicines for people with serious diseases. Kenyon — who is a mechanical engineer by training with over 15 years of experience combining computational and experimental work — says Regeneron has made great strides in simulation by growing from small successes.
One of those successes is in drop testing of wearable electro-mechanical drug delivery devices.
“If we went the traditional route of developing a device, dropping it, figuring out what went wrong, and fixing it, then we would already be making design and manufacturing decisions,” he says. “You’re constrained. You’ve already made prototypes and molds; the arrangement of internal components has been fixed. If something fundamentally needs to be changed, there are severe limitations on what can be done. Doing virtual drop testing early in the process is so much easier than creating and redesigning physical prototypes.”
Simulation-led Design Supports Ideation
Ansys Mechanical plays a big role in Regeneron’s device development efforts.
“Even as we establish feasibility, Ansys simulation is guiding that effort,” Kenyon says. “Simulation is intimately tied to ideation.”
For example, Regeneron is using Mechanical to analyze the interaction of a plunger inside a glass cartridge, both of which are components in a drug delivery device design. With simulation, the company can model the plunger properties, study friction models, and assess plunger rod deformation to predict leakage margins. The company also employs simulation for thermal analysis to evaluate device robustness to various temperature ranges and predict how the device’s container closure will behave. Those results are fed into systems models to establish boundary conditions that are applied to the Mechanical simulation.
While every device is different, Kenyon says using simulation upfront to develop new devices, rather than just to verify a physical prototype, has enabled Regeneron to drastically reduce the time and expense involved in product development.
“When you design a complex device, you go through different phases of engineering and design,” he says. “Instead of having to do three or four of those iterations, with simulation, we can do it in one or two. Because we have simulation, we can get into things that we fundamentally couldn’t do before, so we’ve reduced the number of iterations, but we’ve also developed products that have more capabilities.”
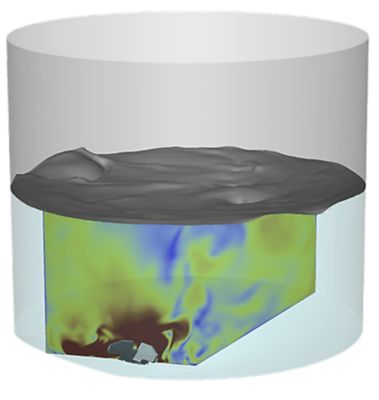
Regeneron uses Ansys for fluid analyses in mixing and is developing a formal validation of its mixing simulation process.
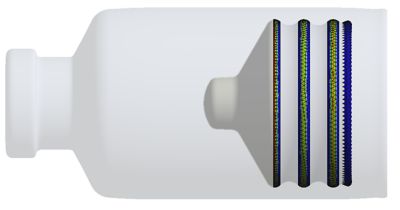
Regeneron is using Ansys Mechanical to analyze the interaction of a plunger inside a glass cartridge by modeling the plunger properties, employing friction models, and assessing plunger rod deformation to predict leakage margins.
A Mixture of Trust, But Verify
Regeneron is also using Ansys computational fluid dynamics (CFD) software for fluid analyses in mixing. As you can imagine, there are many mixing applications involved in the development of medicine. Mixing is conducted throughout the manufacturing process and with the final product that is shipped out in frozen containers that are thawed, pooled together, and mixed.
“Traditionally the way we approach process design and process validation is to run an array of tests to verify that mixing is generating a homogenous mixture and not damaging any of the raw materials used in the process,” Kenyon says. “We started to introduce simulation by answering little questions that came up here and there that weren’t covered in a test matrix.”
Kenyon says he built trust in simulation by being able to answer those questions. Regeneron is now at the point of developing a formal validation of its mixing simulation process. “I wouldn’t say we’re at the point of eliminating physical testing,” he says, “but certainly we’re able to reduce the amount of physical testing and realize time and money savings with simulation.”
Regeneron’s use of simulation continues to grow. The company now simulates challenges large and small, including in silico smoke studies of entire facilities that must be certified as sterile environments. Understanding airflow in such facilities is one of the requirements for certification. Traditionally, this meant building the facility, installing the airflow equipment, and doing a physical smoke study.
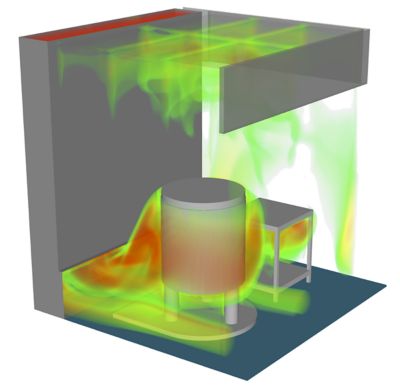
Regeneron uses Ansys simulation to perform smoke studies of entire facilities that have to be certified as sterile environments.
“If you have an issue with a physical smoke study, there are only so many levers you can pull,” Kenyon says. “We haven’t implemented this yet, but we’ve demonstrated what can be done with CFD to assess a smoke study before a facility gets built.”
Communication is Key to Digital Transformation
From small successes to airflow studies of entire facilities, simulation has become a key component in Regeneron’s digital transformation roadmap.
Founded and led for nearly 35 years by physician-scientists, Regeneron's unique ability to repeatedly and consistently translate science into medicine has led to numerous FDA-approved treatments and product candidates in development, almost all of which were homegrown in Regeneron's laboratories. The company is investing in the IT infrastructure it needs for the future, including cloud computing, which runs in tandem with efforts to digitize process and manufacturing data.
“Right now, simulations inform the development process, but we see the possibility for them to take more of a front seat in our validation activities,” Kenyon says. “Leadership support is key to this kind of thing. We are lucky to have strong champions in the business.”
Kenyon emphasizes the importance of communication and collaboration in the digital transformation journey.
“There are a lot of different groups involved in the manufacturing process. You need to have a well-defined communications plan,” he says. “You have to be willing and able to educate people who aren’t familiar with simulation technology.”
The key is making a business connection to the simulation application.
“We need to learn the business process from them, and they need to learn the simulation process from us,” he says. “We have to bridge that gap.”
Learn more about Ansys healthcare enigneering simulation solutions.










