-
United States -
United Kingdom -
India -
France -
Deutschland -
Italia -
日本 -
대한민국 -
中国 -
台灣
-
-
产品组合
查看所有产品Ansys致力于通过向学生提供免费的仿真工程软件来助力他们获得成功。
-
ANSYS BLOG
April 27, 2022
The one thing to remember about battery selection is that there is no such thing as a perfect battery that works for every application. Selecting the right battery for your application is about identifying the most important battery metrics and trading these off against others. For instance, if your application needs a lot of power, internal cell resistance needs to be minimized; this is often done by increasing electrode surface area. However, this also increases inactive components such as current collectors and conductive aid, so energy density is traded off to gain power.
While your actual design goals on the battery may be lofty, you may have to give up some of your goals to achieve others when it comes to actual battery performance (Figure 1).
In this three-part blog series, we will learn that finding the right battery for your application is all about making the right trade-offs. Part one discusses the important considerations when selecting the right battery for a consumer application. These include rechargeability, energy density, power density, shelf life, safety, form factor, cost, and flexibility. Part two discusses how chemistry affects important battery metrics, and therefore battery selection. In part three, we will look at common secondary battery chemistries.
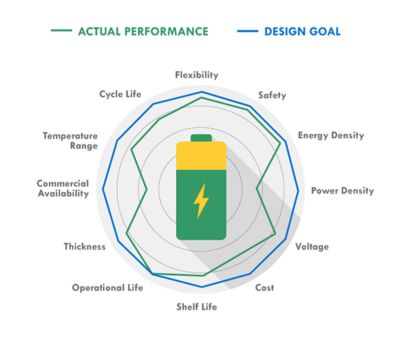
Figure 1. Battery design vs. performance
Important Considerations in Battery Selection
When determining which battery to use, make sure you consider these four factors.
1. Primary vs. Secondary
One of the first choices in battery selection is to decide whether the application requires primary (single use) or secondary (rechargeable) batteries. For the most part, this is an easy decision for the designer. Applications with occasional intermittent use (such as smoke alarms, toys, or flashlights) and disposable applications in which recharging becomes impractical (such as hearing aids, watches, greeting cards, and pacemakers) warrant the use of a primary battery. If the battery is used continuously and for long stretches of time (such as in a laptop, cell phone, or smart watch), a rechargeable battery is more suitable.
Primary batteries have a much lower self-discharge rate — an attractive feature when charging is not possible or practical before first use. Secondary batteries tend to lose energy at a higher rate. This is less important in most applications because of recharging capabilities.
2. Energy vs. Power
The runtime of a battery is dictated by the battery capacity expressed in milliampere per hour (mAh) or amp hours (Ah) and is the discharge current that a battery can provide over time.
Open-circuit voltage is commonly used in energy calculations — i.e., battery voltage when not connected to a load. However, capacity and energy are heavily dependent on the drain rate. Theoretical capacity is dictated only by active electrode materials and active mass, yet practical batteries achieve only a fraction of the theoretical numbers due to the presence of inactive materials and kinetic limitations. These prevent full use of active materials and create buildup on the electrodes.
Battery manufacturers often specify capacity at a given discharge rate, temperature, and cutoff voltage. The specified capacity will depend on all three factors. When comparing manufacturer capacity ratings, make sure you look at drain rates in particular. A battery that appears to have a high capacity on a spec sheet may actually perform poorly if the current drain for the application is higher. For instance, a battery rated at 2 Ah for a 20-hour discharge cannot deliver 2 A for one hour, but will only provide a fraction of the capacity.
Batteries with high power provide rapid discharge capability at high drain rates, such as in power tools or automobile starter batteries. Typically, high-power batteries have low energy densities.
A good analogy for power versus energy is to think of a bucket with a spout. A larger bucket can hold more water and is akin to a battery with high energy. The opening or spout size from which the water leaves the bucket is akin to power — the higher the power, the higher the drain rate. To increase energy, you would typically increase the battery size, but to increase power you decrease internal resistance. Cell construction plays a huge part in obtaining batteries with high power density.
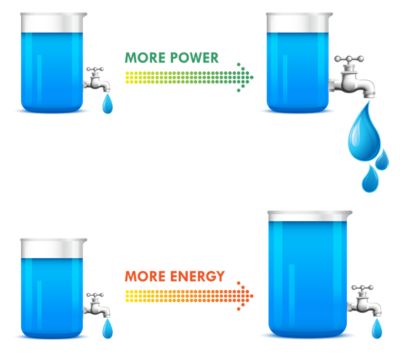
Figure 2. Battery energy vs. power
You can compare theoretical and practical energy densities for different chemistries from battery textbooks. However, because power density is so heavily dependent on battery construction, you will rarely find these values listed.
3. Voltage
Battery operating voltage is another important consideration and is dictated by the electrode materials used. A useful battery classification here is to consider aqueous or water-based batteries versus lithium-based chemistries. Lead acid, zinc carbon, and nickel metal hydride (NiMH) all use water-based electrolytes and have nominal voltages ranging from 1.2 to 2 V. Lithium-based batteries, on the other hand, use organic electrolytes and have nominal voltages of 3.2 to 4 V (both primary and secondary).
Many electronic components operate at a minimum voltage of 3 V. The higher operating voltage of lithium-based chemistries enables a single cell to be used rather than two or three aqueous-based cells in series to make up the desired voltage.
Another thing to note is that some battery chemistries such as zinc manganese dioxide (Zn/MnO2) have a sloping discharge curve, while others have a flat profile. This influences the cutoff voltage (Figure 3).
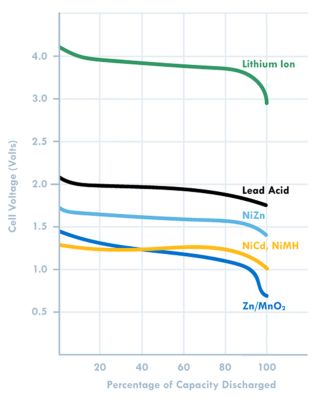
Figure 3. Voltage plot based on battery chemistry
4. Temperature Range
Battery chemistry dictates the temperature range of the application. For instance, aqueous electrolyte-based zinc-carbon cells cannot be used below 0° C (32° F). Alkaline cells also exhibit a sharp decline in capacity at these temperatures, although less than zinc-carbon. Lithium primary batteries with an organic electrolyte can be operated up to -40° C/F, but with a significant drop in performance.
In rechargeable applications, lithium-ion batteries can be charged at maximum rate only within a narrow window of about 20-45° C (68°-113° F). Beyond this temperature range, lower currents/voltages need to be used, resulting in longer charging times. At temperatures below 5-10° C (41-50° F), a trickle charge may be required to prevent the dreaded lithium dendritic plating problem, which increases the risk of thermal runaway. We have all heard of exploding lithium-based batteries, which could happen as a result of overcharging, low- or high-temperature charging, or short-circuiting from contaminants.
Other Battery Considerations
In addition to the four main considerations listed above, the fundamental factors listed below come into play when selecting a battery for a particular application.
Shelf Life
How long a battery will sit in a storeroom or on a shelf before it is used may be an important consideration. Primary batteries have much a longer shelf life than secondary batteries. However, shelf life is generally more important for primary batteries because secondary batteries have the ability to be recharged. An exception is when recharging is not practical.
Chemistry
Many of the properties listed above are dictated by cell chemistry. We will discuss commonly available battery chemistries in the next part of this blog series.
Physical Size and Shape
Batteries are typically available in different size formats — such as button/coin cells, cylindrical cells, prismatic cells, and pouch cells (most of them are standardized).
Cost
There are times when you may need to pass up a battery with better performance characteristics because the application is very cost sensitive. This is especially true for high volume disposable applications.
Transportation and Disposal Regulations
Transportation of lithium-based batteries is regulated. Disposal of certain battery chemistries is also regulated. This may be a consideration for high-volume applications.
As you can see, there are many considerations when selecting a battery. Several of these are related to chemistry, while others are related to battery design and construction. This makes it harder and sometimes meaningless to do a battery metric comparison without a more fundamental understanding of the factors that affect that metric — a topic we’ll explore in the second blog of this series.
Want to improve the battery reliability of your product? Request a quote here.










