-
United States -
United Kingdom -
India -
France -
Deutschland -
Italia -
日本 -
대한민국 -
中国 -
台灣
-
-
产品组合
查看所有产品Ansys致力于通过向学生提供免费的仿真工程软件来助力他们获得成功。
-
Ansys Blog
March 13, 2017
3 Milestones on Our Way to the Medical Digital Twin: Ansys Leads the Charge
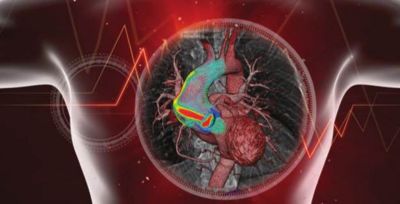
In September 2016, I wrote about the medical digital twin concept. I continue to read numerous articles showing confidence that we are indeed on our way towards the medical digital twin. One particular article from the BBC nicely described how our heart digital twin could prevent its failure.
If everybody agrees this is the direction we need to follow, many think that the medical digital twin is a concept way ahead in the future.
I slightly disagree as there are 3 major on-going initiatives paving the way to the medical digital twin likely to reach key milestones in the foreseeable future:
- Personalized Medicine to provide the necessary information to the digital twin
- Complete Digital Prototypes to accelerate the emergence of this new medicine
- In silico Clinical Trials to get innovations approved faster through
Personalized Medicine
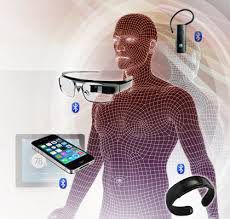
Medicine is becoming personalized. Soon, all of us will be under permanent intense care. All our vital and non-vital parameters will be continuously monitored. But the bioelectronic market still need 5 key innovations.
1. Wearable monitoring and diagnosis equipment requires measurement of target parameters in a reliable and repetitive way
2. Energy efficiency to prevent the replacement or recharge of batteries
3. Signal interference, signal integrity and cyber security of wearables
4. Patient safety and compliance with strict regulations
5. Software and a simple interface that would be able to treat patient data and synthesize them in crisp recommendations for the patient
Although this list may look intimidating, amazing progresses are reported every day. Innovation should just be faster.
Complete Digital Prototype
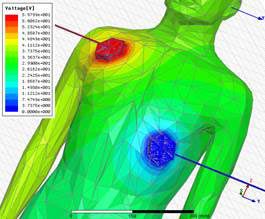
Whether this is for designing the next wearable, new drug delivery processes or simply hospital beds, the product development process is often “under-digitized”. Computer Model and Simulation (CM&S) is used pervasively to cost effectively design products across all industries including leading medical devices companies that have reported dramatic acceleration and return on investment exceeding 1 to 30. There are a few reasons why smaller companies have not yet fully embraced complete digital prototypes:
Testing defibrillator of
Human Body Modeling
- Lack of skilled employees fully educated in engineering simulation; I recently discussed the need for bioengineering education
- Lack of confidence that simulation significantly reduces time and cost to market, possibly minimizing the risk for the patient, although widely reviewed in the literature.
- Despite convinced by the value of simulation and its ROI some people keep delaying the necessary investment : the competitors and the patients can’t wait!
Fortunately, every year more and more companies adopt simulation.
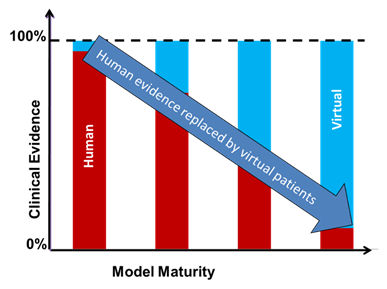
In silico Clinical Trials
The long and painful, but necessary, regulatory approval process is really what is preventing innovations to reach the patients faster. Conscious of this problem, but unwilling to compromise with patients’ safety, legislators and regulators are looking for alternative solutions such as complementing clinical trials with preliminary tests on computers, a.k.a. in silico clinical trials.
But we are still facing some difficulties:
- Building the medical community confidence through extensive clinical validations
- Large libraries of patient specific geometries, materials properties and pathologies
- Definition of the formal regulatory frame by legislation
Verification and Validation (V&V 40)
Getting enough confidence in engineering simulation for healthcare applications is the primary challenge on the way to medical digital twin. I’d like to acknowledge the phenomenal work of a group of medical and modeling experts under the supervision of the FDA which is defining the concrete steps necessary to properly verify that the models properly solve the equations and validate that these computer models adequately represent the reality.
The “Verification and Validation 40” document will be released within the next few months. It will facilitate the large scale adoption of complete digital prototypes that will accelerate the pace of innovation and reduce the time for regulatory approval making personalized medicine a reality faster than through a traditional approach.