ARGOMENTO IN DETTAGLIO
Cos'è il trasferimento di calore?
Il trasferimento di calore è il movimento dell'energia termica, sotto forma di calore, tra sistemi fisici con temperature diverse utilizzando quattro meccanismi distinti: avvezione, conduzione, convezione e radiazione. Il trasferimento di calore ha un impatto su quasi ogni aspetto della vita moderna, dalla cottura del cibo al raffreddamento di un laptop alla generazione di elettricità in una centrale elettrica. Ogni giorno, il riscaldamento della Terra dovuto alla radiazione solare del sole è un processo di trasferimento di calore, proprio come i temporali sono causati dalle correnti convettive nell'atmosfera terrestre.
Il trasferimento di calore avviene come un fenomeno naturale in qualsiasi situazione in cui vi siano differenze di temperatura tra due sistemi, come descritto nella seconda legge della termodinamica. Gli ingegneri utilizzano anche dispositivi di trasferimento del calore per spostare l'energia sotto forma di calore da un luogo a un altro, fornendo calore dove necessario e rimuovendolo dove può causare problemi. La sicurezza, l'efficienza e le prestazioni dei prodotti sono garantite dal mantenimento della temperatura di ciascuna parte del prodotto entro un intervallo di temperature desiderato.
Fondamenti del trasferimento di calore
Il trasferimento di calore riguarda fondamentalmente lo spostamento di energia sotto forma di calore da un sistema a un altro. La disciplina esamina la quantità di energia che viene immagazzinata o trasferita sotto forma di calore e i diversi modi in cui l'energia viene spostata attraverso i sistemi.
Di seguito alcuni termini importanti utilizzati nel trasferimento di calore e il loro significato.
Correnti di convezione
Le correnti di convezione si riferiscono a un fluido che vede un aumento locale della temperatura e una corrispondente diminuzione della densità a causa del riscaldamento. Il calo di densità crea una spinta ascensionale che fa sì che il fluido più caldo salga fino a quando non si raffredda. Quindi, quando le regioni si raffreddano, la densità diminuisce e la regione fluida viene spinta verso il basso dalla galleggiabilità negativa. Queste correnti possono essere facilmente viste in una pentola di spaghetti in ebollizione, dove le correnti convettive fanno attorcigliare e girare la pasta nell'acqua.
Capacità termica o massa termica
La capacità termica di un sistema è la quantità di calore necessaria per aumentare la temperatura del sistema di un grado. Maggiore è la capacità termica, maggiore è l'energia necessaria per aumentare la sua temperatura. È un valore importante nel trasferimento di calore perché rivela come i materiali rispondono al calore e quanto velocemente l'oggetto può riscaldarsi o raffreddarsi.
Flusso di calore
Il flusso di calore misura la quantità di energia trasferita attraverso un'unità di area. È il calore totale diviso per l'area della superficie su cui viene misurato. È un valore critico nel trasferimento di calore perché indica agli ingegneri la quantità di energia che viaggia da un oggetto a un altro o da un oggetto a un fluido.
Generazione di calore
La generazione di calore è la creazione di energia termica attraverso molteplici processi, tra cui reazioni chimiche, combustione, fusione nucleare, fissione nucleare, termoelettrici, resistenza elettrica, attrito meccanico o variazioni di pressione in un fluido.
Coefficiente di trasferimento di calore
Il coefficiente di trasferimento di calore (h) di una superficie misura la relazione tra il flusso di calore tra una superficie e il fluido che tocca la superficie e la differenza di temperatura tra la superficie e il fluido. Il valore può essere calcolato se si conoscono la velocità e le proprietà termiche del fluido. A volte viene chiamato coefficiente di scambio termico.
Trasferimento di massa
Proprio come il trasferimento di calore riguarda il movimento del calore da un sistema all'altro, il trasferimento di massa riguarda il movimento della massa. Questo è importante nel trasferimento di calore perché qualsiasi massa che si muove tra i sistemi ha un'energia termica interna che si muove con la massa. L'aria che si muove su un dissipatore di calore è sia un trasferimento di massa sia un trasferimento di calore.
Cambio di fase
Quando la materia passa da uno stato a un altro, subisce un cambiamento di fase. Tecnicamente, il cambiamento di fase non è un trasferimento di calore, ma viene utilizzato con il trasferimento di calore per controllare le temperature nei sistemi. I cambiamenti nei legami molecolari all'interno della fase di cambiamento del materiale assorbono energia se si passa da solido a liquido (fusione) o da liquido a gas (ebollizione o vaporizzazione) e rilasciano energia se si passa da gas a liquido (condensazione) o da liquido a solido (congelamento). Gli ingegneri utilizzano spesso il cambiamento di fase per rimuovere o aggiungere calore a un sistema.
Temperatura
La temperatura è una misura dell'energia cinetica degli atomi e delle molecole che vibrano e si scontrano in un oggetto.
Conduttività termica
La conduttività termica (k) di un materiale è una misura della capacità di quel materiale di condurre il calore. L'inverso della conduttività termica è la resistività termica.
Energia termica o calore
Il calore è l'energia interna della materia che assume la forma del movimento microscopico di particelle subatomiche, atomi e molecole. Maggiore è la temperatura, maggiore è l'energia. Per essere precisi, il calore si riferisce solo alla quantità di energia termica trasferita. Tuttavia, la maggior parte delle persone utilizza il calore e l'energia termica in modo intercambiabile.
Equilibrio termico
L'equilibrio termico è lo stato in cui due oggetti a contatto hanno la stessa temperatura. Quando viene raggiunto l'equilibrio termico, il trasferimento di calore tra i due oggetti si interrompe.
Termodinamica
La termodinamica è una branca della fisica focalizzata sul calore, il lavoro e la temperatura e su come questi influenzano l'energia e l'entropia dei sistemi e le proprietà fisiche della materia. È descritta dalle quattro leggi della termodinamica. La fisica del trasferimento di calore è la parte della termodinamica che si occupa del flusso di calore tra gli oggetti.
Le quattro modalità di trasferimento del calore
Il movimento dell'energia termica tra i sistemi può avvenire in una delle quattro modalità, in cui l'energia viene trasferita quando il calore scorre dall'oggetto a temperatura più elevata all'oggetto a temperatura più bassa. Nella maggior parte dei casi sono attivi contemporaneamente due o più tipi di trasferimento del calore, a seconda che gli oggetti coinvolti siano in contatto, circondati da un fluido o visibili tra loro.
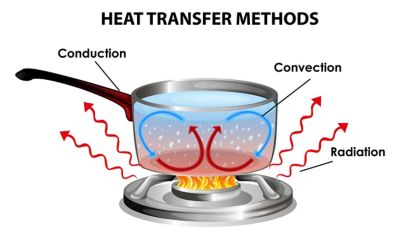
Ecco una breve definizione di ciascuna modalità.
Avvezione
L'avvezione termica è il meccanismo di trasferimento di energia termica in cui il calore viene trasportato da un luogo a un altro attraverso il movimento e la quantità di moto di un fluido. L'avvezione viene talvolta definita convezione forzata per differenziarla dalla definizione rigorosa di convezione perché il flusso del fluido in avvezione non è causato da forze di galleggiamento, ma è invece impartito aggiungendo energia al sistema.
Una ventola che raffredda la scheda madre di un computer è un esempio di trasferimento di calore avvettivo.
Conduzione termica
La conduzione termica descrive il trasferimento di calore tra due oggetti a diretto contatto o all'interno di un oggetto con una differenza di temperatura attraverso l'oggetto. Descrive il trasferimento di energia attraverso la diffusione termica come descritto nella legge di Fourier per la conduzione del calore. La velocità del trasferimento di energia è determinata dalla conduttività termica del materiale e dal gradiente di temperatura nell'oggetto o negli oggetti. Per due oggetti in contatto fisico, la pressione e l'adattamento tra le due superfici determinano la resistenza al contatto termico.
Un esempio di conduzione termica è il manico di una pentola sul fornello. Il calore si sposta dalla base della pentola alle pareti e al manico.
Convezione
La convezione, o trasferimento di calore convettivo, è il trasferimento di energia termica dovuto al movimento di un fluido guidato dalla galleggiabilità causata dalle differenze di temperatura nel fluido. Gli ingegneri generalmente la chiamano convezione libera o convezione naturale per differenziarla dall'avvezione o dalla convezione forzata.
Un esempio comune di convezione è semplicemente lasciare una tazza di caffè o tè caldo all'aria. La bevanda calda trasmette il calore nell'aria e le forze ascensionali lo disperdono dell'ambiente.
Radiazioni
Il trasferimento di calore per radiazione è un meccanismo che trasferisce l'energia termica sotto forma di onde/fotoni elettromagnetici. L'energia termica fa sì che gli atomi di qualsiasi forma di materia si muovano e il movimento delle particelle cariche in quegli atomi (protoni ed elettroni) provoca l'emissione di radiazioni elettromagnetiche. Il trasferimento di calore dovuto alla radiazione termica avviene solo nel vuoto o attraverso un mezzo trasparente alle lunghezze d'onda degli infrarossi emesse a causa della temperatura di un oggetto.
Applicazioni di trasferimento di calore
L'energia sotto forma di calore può essere utilizzata per svolgere lavoro, oppure può avere un impatto negativo su qualsiasi sistema a causa delle differenze di temperatura. I fondamenti del trasferimento di calore vengono applicati dagli ingegneri per controllare la quantità di energia termica che entra nei sistemi, spostare l'energia termica come desiderato all'interno dei sistemi e rimuovere l'energia termica dai sistemi utilizzando uno o più meccanismi di trasferimento.
Ecco un elenco di alcune delle applicazioni più comuni del trasferimento di calore.
Cucinare
Preparazione del cibo attraverso l'applicazione del calore per renderlo sicuro e commestibile. Il fuoco era originariamente utilizzato come fonte di calore, dove una combinazione di convezione e trasferimento di calore per radiazione sposta il calore dal combustibile in combustione al cibo.
Nel corso del tempo, furono sviluppati forni per creare ambienti ad alta temperatura dove la convezione dell'aria riscaldata e l'irraggiamento delle pareti cuocevano il cibo. La cucina moderna può utilizzare la resistenza elettrica o l'induzione elettrica come fonte di calore per trasferire energia nei recipienti di cottura. Le friggitrici e i forni a convezione sono esempi di utilizzo dell'avvezione sotto forma di ventilatori che soffiano aria ad alta temperatura sul cibo per aumentare il flusso di calore nel cibo e cuocerlo più velocemente.
Gestione termica elettronica
Un'altra applicazione molto comune del trasferimento di calore nella vita moderna è il raffreddamento dei dispositivi elettronici. La resistenza nei componenti elettronici genera calore e vengono utilizzati vari metodi di trasferimento del calore per rimuovere il calore dai componenti.
Nella sua forma più elementare, come in un telefono cellulare, la conduzione viene utilizzata per trasferire l'energia termica dai componenti alla custodia e allo schermo, dove la convezione trasferisce il calore all'aria circostante. Per i dispositivi a temperature più elevate come i computer, i dissipatori di calore sono progettati per creare un'ampia superficie per la convezione e, se necessario, viene utilizzata l'avvezione posizionando ventole nel dispositivo per aumentare la quantità di moto dell'aria, allontanando il calore e aumentando il coefficiente di scambio termico.
Riscaldamento e raffreddamento
Gli esseri umani hanno utilizzato il trasferimento di calore anche per progettare dispositivi e materiali che mantengano una temperatura confortevole all'interno delle strutture:
- Gli edifici utilizzano l'isolamento per ridurre il trasferimento di calore dall'interno della struttura all'ambiente esterno.
- I rivestimenti sulle finestre riducono la quantità di radiazione infrarossa che passa attraverso le finestre e i sistemi di condizionamento dell'aria utilizzano il cambiamento di fase per estrarre calore dall'aria e restituire aria a bassa temperatura alle stanze.
- Per il riscaldamento, si utilizzano la combustione o l'energia elettrica per riscaldare aria o vapore che trasportano il fluido riscaldato dove serve.
Lavorazione dei materiali
Dalla produzione di leghe metalliche all'estrazione di prodotti petroliferi dal petrolio greggio, il trasferimento di calore svolge un ruolo essenziale nella maggior parte degli esempi di lavorazione dei materiali.
In ciascun caso, il trasferimento di calore viene utilizzato per ottenere e mantenere la temperatura desiderata nella materia prima per creare il cambiamento di fase, la reazione chimica o la modifica metallurgica desiderata. Il trasferimento di calore è studiato e utilizzato per ottimizzare l'efficienza della lavorazione dei materiali per ridurre al minimo la quantità di energia necessaria.
Motori di autoveicoli
I motori a combustione interna (ICE) creano una quantità significativa di calore. Sebbene alcuni motori facciano affidamento sul raffreddamento ad aria, la maggior parte utilizza acqua pompata per estrarre calore dal blocco motore, che è realizzato in acciaio o alluminio, entrambi conduttori di calore.
Il fluido viene quindi fatto passare attraverso un radiatore, un grande scambiatore di calore nella parte anteriore dell'auto, per trasferire l'energia termica attraverso l'avvezione all'aria utilizzando la velocità dell'auto quando è in movimento o i ventilatori quando è ferma o si muove lentamente.
Motopropulsori per veicoli elettrici
I motori e le batterie dei veicoli elettrici (EV) possono generare una notevole quantità di calore che deve essere trasferito all'esterno del veicolo. Alcuni sistemi sono progettati per utilizzare la conduzione per allontanare il calore dalla fonte termica e quindi utilizzare circuiti di raffreddamento del liquido per trasportare il calore ai radiatori.
Veicoli spaziali
Il raffreddamento e il riscaldamento dei componenti elettronici nello spazio, e in particolare dei sensori come le fotocamere CMOS sui veicoli spaziali, rappresentano un problema di trasferimento di calore unico perché non c'è aria circostante a cui trasferire l'energia termica. Il progetto deve bilanciare il calore generato dall'elettronica, la radiazione infrarossa proveniente dall'ambiente, il calore assorbito dal sole e il calore irradiato nello spazio. Per mantenere la temperatura dei componenti nello spazio entro il corretto intervallo operativo, gli ingegneri utilizzano una combinazione di conduzione, radiazione, avvezione, generazione di calore o cambiamento di fase.
Raccomandazioni per la simulazione del trasferimento di calore
Gli ingegneri che progettano sistemi di gestione termica fanno molto affidamento sulla simulazione per comprendere i sistemi che stanno sviluppando e per guidare i loro progetti. La simulazione può essere notevolmente accelerata, eseguita nelle prime fasi del processo di progettazione ed esplorare molti più scenari rispetto ai test fisici.
In alcuni casi, la simulazione può assumere la forma di poche equazioni. Man mano che i sistemi diventano più complessi, gli ingegneri utilizzano l'analisi degli elementi finiti (FEA), l'analisi delle differenze finite, la fluidodinamica computazionale (CFD) e il ray tracing per modellare le modalità di conduzione, convezione/avvezione e radiazione del trasferimento di calore.
Ecco alcuni suggerimenti per condurre simulazioni accurate ed efficienti del trasferimento di calore.
1. Determinare se e quando è necessaria una soluzione transitoria o stazionaria
Il trasferimento di calore è un fenomeno dipendente dal tempo perché l'energia termica impiega tempo per spostarsi tra gli oggetti fino al raggiungimento dell'equilibrio termico. Prima di iniziare una simulazione, bisognerebbe capire se è necessario acquisire il comportamento transitorio del sistema o se si è interessati solo ai flussi di calore e alle temperature una volta soddisfatte le condizioni di stato stazionario.
2. Conoscere le proprietà dei materiali
I calcoli accurati del trasferimento di calore dipendono fortemente dalle proprietà del materiale come conduttività termica, capacità termica ed emissività. Utilizzare uno strumento come Ansys Granta per ottenere e gestire le proprietà termiche dei materiali può essere molto utile.
3. Comprendere le condizioni al contorno e i volumi di controllo
Una delle fonti di errore più comuni nella simulazione del trasferimento di calore è l'applicazione di condizioni al contorno errate o la mancata definizione del volume di controllo adeguato attorno agli oggetti. Prestare attenzione a disegnare ogni regione del sistema che si sta modellando e assicurarsi di conoscere il flusso di calore e la generazione di calore in ciascuna di esse prima di costruire il modello.
4. Acquisizione accurata delle velocità dei fluidi
La convezione naturale e forzata sono le due modalità più comuni di trasferimento di calore utilizzate per gestire il flusso di calore nei sistemi e il flusso termico da una superficie a un fluido dipende fortemente dalla velocità.
Un programma FEA come il software di analisi strutturale agli elementi finiti Ansys Mechanical o un programma alle differenze finite come il software di modellazione termocentrica Ansys Thermal Desktop può rappresentare il trasferimento di calore convettivo a un fluido utilizzando un coefficiente di trasferimento di calore se la velocità è ben nota. Se necessario, il fluido può essere semplificato come una rete termofluida 1-D per calcolare con maggiore precisione il trasferimento di calore e la velocità dei fluidi nel sistema. Questa è una pratica comune nei motori automobilistici, nei motori a reazione, nelle centrali nucleari e nei veicoli spaziali.
Tuttavia, nei sistemi complessi in cui il flusso dei fluidi non può essere stimato o semplificato, gli ingegneri utilizzano uno strumento CFD completo come il software Ansys Fluent per prevedere con precisione il flusso dei fluidi, comprese turbolenze, correnti convettive e miscelazione di fluidi diversi. Le simulazioni che combinano un'accurata conducibilità termica nei solidi e il comportamento dei fluidi sono chiamate simulazioni di trasferimento di calore coniugato.
5. Semplificare i modelli quando possibile
Gli ingegneri termotecnici di grande esperienza sono esperti nella semplificazione dei sistemi per la simulazione del trasferimento di calore. In molti casi, è possibile utilizzare un modello di rete 1-D per catturare con precisione alcuni o tutti i comportamenti termici di un sistema. Oppure, la geometria 3D può essere notevolmente semplificata perché piccole caratteristiche non influiscono sulla risposta al trasferimento di calore.
Uno strumento come Thermal Desktop può creare modelli semplici per i primi studi di progettazione prima che la geometria CAD sia disponibile e mantenere una semplificazione semplificata non appena la geometria CAD diventa disponibile con TD Direct in Ansys SpaceClaim.
Uno strumento come TD Direct in Ansys SpaceClaim può accelerare notevolmente la conversione di geometrie CAD complesse in un modello semplificato ideale per l'analisi termica transitoria.
6. Sfruttare la potenza delle applicazioni verticali per l'elettronica
La modellazione del trasferimento di calore nell'elettronica è una sua specialità, quindi sono stati sviluppati strumenti come il software di simulazione del raffreddamento dell'elettronica Ansys Icepak per concentrarsi su questa importante area. Le applicazioni verticali comprendono la terminologia, la geometria e le condizioni al contorno specifiche del settore e includono modi per automatizzare le fasi di creazione del modello e post-elaborazione. L'utilizzo di un'applicazione verticale non solo fa risparmiare tempo, ma consente anche ai non esperti di utilizzare più facilmente la simulazione.










