ANSYS ADVANTAGE MAGAZINE
October 2021
Digital Twins Help Bioreactors to Produce Personalized, Cell-Based Therapies
By Ansys Advantage Staff
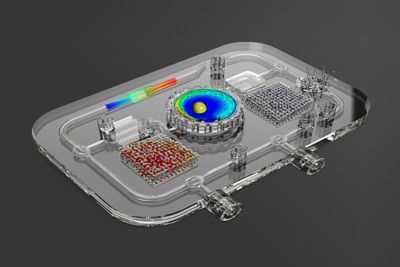
While a few big pharma companies produced amazing results in developing and manufacturing massive amounts of COVID-19 vaccines in under a year, many pharma challenges exist on a smaller scale — that of an individual patient or small group of patients with a rare disease. This “personalized medicine” approach has led companies like Antleron in Leuven, Belgium, to redefine the concept of a bioreactor, using Ansys simulation solutions and digital twins to optimize and customize the growth of cells for therapeutic purposes.
“Though we still use the word ‘bioreactor,’ I think a better description is ‘customized factory,’” says Jan Schrooten, co-founder and CEO of Antleron. “It looks like a chemical plant in miniature, and the output might be 1 milligram of end product, instead of thousands of liters in a large pharma plant.”
Schrooten founded Antleron in 2014 after working in academia for 25 years trying to build living implants and experimenting with tissue engineering. Being an engineer and faced with the limitations of academia in translating lab work into real-world solutions, he quit his university position and founded a startup company. After much thought, he and his colleagues decided to call the company Antleron after the way deer antlers grow from nothing to a meter or more in length every year — one of the most impressive regenerative biological processes in nature. The name fits their mission of “Enabling living therapies: turning cells into personalized therapies, tissues and organs.”
Help from the Ansys Startup Program
Schrooten was no stranger to Ansys simulation products, having worked with them for about 25 years, and he recognized that simulations would be essential to engineering and monitoring the bioreactors he had in mind. But, like most startups, funds were not exactly plentiful at the beginning. So, he contacted Ansys and learned about the Ansys Startup Program, which provided him with simulation software at a fraction of the list price. But he sees more than just financial value in the program.
“Even if we could have paid for a full software license, it wouldn’t have been as helpful as going through the Ansys Startup Program,” Schrooten says. “Exploring the software with Ansys experts and getting their feedback about how best to do what we were attempting is the key. The program provided a framework for our companies to learn from each other and develop a strong relationship that benefits both of us. By engaging in similar collaborations with other partners, we are able to merge the potential of various core computational and experimental techniques and integrate them to enable engineering of innovative cell-based therapy manufacturing solutions.”
Using Ansys solutions, Antleron has been able to design custom bioreactors that place living cells in the best environment for growth and modification. They use Quality By Design, digital twins, additive manufacturing and other engineering technologies to achieve success.
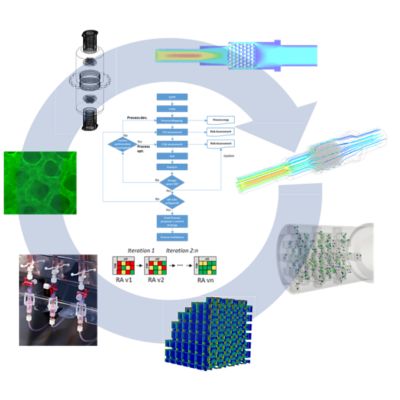
Digital twins are part of Antleron’s Quality-by-Design approach to fast-track development of optimized and scalable bioreactor designs. In an iterative risk-based development process loop, designs are first tested in silico by CFD simulations. Then promising designs are additively manufactured and experimentally tested, resulting in optimized, robust and scalable bioreactor designs at a minimal time and cost.
Simulating the Complexities of Bioreactors
A quick internet search of images of bioreactors yields a series of tanks, stirrers, piping, valves and more that resemble any generic chemical plant and reveal very little about the complex inner workings of a bioreactor. When you add living cells to the equation, with the inherent complexity and variability of biological processes, things can quickly get out of hand.
“One cell by itself is much more complex than the whole bioreactor that we are working on,” says Tommy Heck, who leads the digital twin and modeling efforts at Antleron. “Inside the cell, you have numerous signaling pathways, consisting of various molecules, that all are affected by the nutrients that the cell receives and the mechanical conditions that the cell experiences. So that’s just too complex to describe with a simple model. Since we don’t know exactly what’s going on there, it’s better to simulate the process in a hybrid simulation approach.”
The process starts with a cell source. This source can be an individual patient whose cells are extracted, grown and modified in a bioreactor and then returned to the patient, or it can be a bank containing lots of cells from different donors. These cells are accustomed to grow under specific conditions (e.g., temperature, pH and mechanical rigidity) provided by the human body. However, often the environment for cells in in vitro experiments resembles a plastic petri dish open to the air, which is not optimal.
“Putting cells in a more physiological environment that they recognize improves the situation,” Schrooten says. “The key is defining that environment. We create a three-dimensional — even four-dimensional, because time is important — controlled, closed environment that we monitor and customize for best results.”
Instead of cells sticking to the bottom of an open petri dish, or a closed stirred tank with cells floating around in a fluid suspension medium while hanging on to microbeads, the controlled, closed environment of a bioreactor can also be filled with a high-surface-area material to give the cells a more customized and controllable surface on which to grow. This internal material could be a highly porous structure or a more engineered 3D pathway designed for optimal fluid flow and cell growth conditions.
“You can often build up the internal bioreactor structure out of geometrical units,” says Heck. “You can perhaps have a repeated cubic shape, or maybe some convex and concave shapes in there, with some larger channels to distribute the fluid flow. Ansys Fluent is great for predicting the flow through these complex paths. By including computational fluid dynamics (CFD) simulations in an iterative loop of design, additive manufacturing and experimentation, we are able to quickly screen and compare various designs. This significantly decreases the time and cost to develop a bioreactor, while its performance is optimized.”
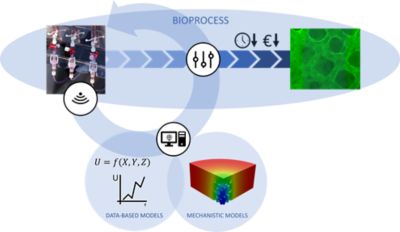
The goal of the combined custom bioreactor with its internal structure is to give the cells a surface to connect to, to provide optimal flow conditions at the same time, and also to ensure efficient supply of the added glucose and oxygen nutrients that feed the cells and make them grow. But inserting flow meters, thermocouples and glucose sensors throughout such a complicated 3D structure is difficult, and therefore the local conditions inside the bioreactor can’t be measured. So Antleron has developed an in silico approach to accommodate co-development of hybrid modeling, with the goal of enabling a digital twin of the bioreactor powered by a combined use of mechanistic, physics-based simulations and a data-based simulation approach.
Hybrid digital twins, combining data-based and mechanistic computer models, facilitate real-time monitoring and control of closed bioprocesses, enabling optimized process outcomes and minimized process time and cost.
"Though we still use the word ‘bioreactor,’ I think a better description is ‘customized factory.’ It looks like a chemical plant in miniature, and the output might be 1 milligram of end product, instead of thousands of liters in a large pharma plant."
— Jan Schrooten, co-founder and CEO of Antleron
A Bioreactor Digital Twin Based on Data and Simulations
“Once you have designed a more optimized bioreactor, you want to be able to know what’s happening during cell culture, so you have to monitor and control the process,” Schrooten says. “But you can’t take the biology out of the bioreactor and perform invasive measurements. You need to put intelligence into the bioreactor and find a way to maximize the use of data. We use digital twins — models that can connect the in silico know-how with the actual experiments — to monitor and control the system in a scalable way.”
The mechanistic simulations in the digital twin, overseen by Heck, are the physics-based simulations of flow rate, flow direction, shear, nutrient concentration and other fluid properties typically done by Fluent. Working on the other half of the digital twin solution, Evan Claes, who is in charge of the advanced therapy medicinal products (ATMP) bioprocess and data-based modeling at Antleron, collects data from sensors inside the bioreactor or from noninvasive sampling to effectively “learn” how the cells in the bioreactor respond to different conditions. “Much like how food intake is monitored for infants or young animals, data on the cells’ nutrient uptake can be used to infer their growth rate — something which would be impossible to directly measure in the bioreactor,” Claes says.
While these data-based models accurately predict cell growth in a “simple” bioreactor, the presence of an engineered internal structure makes the situation more complex. Claes explains: “If you have an internal structure providing a high surface area for the cells to grow on, you may not be able to get a lot of nutrients to the center of this structure — they are consumed by the cells on the outside of the scaffold before they reach the center. But it is almost impossible to gather data on this because you can’t put a sensor inside the structure. However, with Fluent we can simulate the concentration inside the structure from knowledge of the concentration outside the structure, which we can measure.”
Working together, the mechanistic and data-based simulation approaches provide data to feed the digital twin of the bioreactor. The digital twin gives Antleron engineers and scientists a better idea of what is happening in the real bioreactor at a given time, so they can adjust the amount of nutrients, determine how many cells are in the system and know when to stop the process.
Developing Biologic Therapies and Growing Living Tissue
Besides just increasing the number of cells in a bioreactor, biotech companies can also modify the cells to produce cell-based therapeutics. For example, the starting point can be a sample of induced pluripotent stem cells (iPSCs), which are derived from skin or blood cells that have been reprogrammed back into an embryonic-like pluripotent state. From the iPSCs, an unlimited source of any type of human cell needed for therapeutic purposes can be developed.
“Once I have the number of cells that I need, I can transform them into another cell type, or I can combine them with yet another cell type to form a tissue,” Schrooten says. “If I have cells that can form liver or, let’s say, soft tissue, I can give them cues in the bioreactor by combining them with a certain material to be transformed into the end product — in this case, liver.”
Harvesting Cells from Bioreactors
Once the cells are finished growing and have been modified, if necessary, they must be harvested efficiently from the bioreactor. Because each cell is a discrete unit, Rocky DEM, a discrete element software coupled to Ansys Fluent, can help in this process. Rocky DEM can show how changing the flow or adding some enzymes or proteins to the bioreactor can loosen the cells from the substrate they are attached to so they can detach and be harvested.
“You have millions of cells in a bioreactor, but they are separate particles, so you want to treat them as such rather than as a continuous material,” Heck says. “Simulation using Rocky DEM helps us to optimize the conditions for the seeding and harvesting of these cells.”
Current and Future Work
One of Antleron’s current projects involves investigating the potential of immunotherapy to kill cancer cells. Instead of using radiation or chemotherapy, immunotherapy delivers specific proteins that attack the cancer via their attachment to specific cells that are injected into the patient. In this case, the starting point is the patient’s own dendritic cells that are part of their immune system. These cells are multiplied and loaded with the active protein in a bioreactor. Then they are returned to the patient to, hopefully, kill the cancer.
In the long run, Antleron wants to go beyond personalized cell therapies to create living organs that can be transplanted into humans to extend their lives when the original organ fails due to a disease or injury. Despite the number of potential human organ donors, only a small fraction of people actually consent to donating their organs should they die in an accident, and more opportunities are lost because loved ones are not aware of the deceased’s desire to donate organs. Artificial organs could help the supply get closer to the demand, but a lot of challenges remain to reach this lofty goal.
“We see a lot of tools and technologies — for example, additive manufacturing and the power of simulation — being used in other industries that we believe can be helpful in health care,” Schrooten says. “With Ansys simulations, we can do a lot of the development already in silico before we do it in vitro, and then, finally, in vivo. That’s why we say: ‘If we know how a bioprocess works, by simulations and by experiments, we can build the ideal bioreactor around it.’”