-
United States -
United Kingdom -
India -
France -
Deutschland -
Italia -
日本 -
대한민국 -
中国 -
台灣
-
Ansys is committed to setting today's students up for success, by providing free simulation engineering software to students.
-
Ansys is committed to setting today's students up for success, by providing free simulation engineering software to students.
-
Ansys is committed to setting today's students up for success, by providing free simulation engineering software to students.
-
Contact Us -
Careers -
Students and Academic -
For United States and Canada
+1 844.462.6797
ANSYS BLOG
April 14, 2022
Simulation Helps Bring New Drugs to Patients Faster
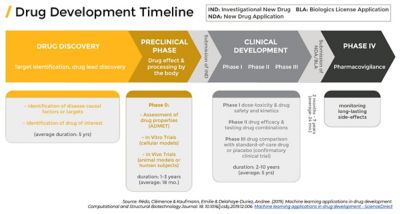
From initial discovery to launch, new drug development typically takes around 12 years and could cost more than a $1 billion for a single drug. It is a very lengthy and expensive process that is constrained by many rules and regulations. Growing costs and timelines mean increased challenges to get innovative treatments and medicine to those who need it most. This has prompted some companies to develop better models to manage new drug development.
Dr. Reddy’s Laboratories, a multinational pharmaceutical company, accelerates access to affordable and innovative medicines by improving the drug manufacturing process with simulation, also known as using in silico methods.
Founded in 1984, Dr. Reddy’s offers generic medicines, active pharmaceutical ingredients (APIs), custom pharmaceutical services, biosimilars, and differentiated formulations. Birendra David is the vice president of delivery manager-inhalation and head of process engineering at Dr. Reddy’s. David and his team participate in the drug development process from introduction to launch. Formulation development requires the product being developed to be optimized on a lab scale and tested on a pilot scale before moving to full production scale. This scale-up process aims to deliver the same critical quality attributes (CQAs) for mass production in laboratory. David and his team are brought in when the product is introduced to Dr. Reddy’s. The process engineering team has the technical capabilities, equipment design knowledge, and familiarity with the right process analytical technology (PAT) tools and quality by design (QbD) principles needed to develop and optimize the product manufacturing process to scale. Dr. Reddy’s has also invested in a dedicated process engineering and modeling lab to develop deeper insights across the scales of production.
“So, we travel the entire length of the product life cycle,” says David, “We are the engineers who participate in the design and entire set up needed to scale up and manufacture the product.”
Before working in the pharmaceutical industry, David was a chemical engineer at Ansys working primarily on oil and natural gas projects. Eventually he started consulting on pharmaceutical projects, which piqued his interest. “The types of simulations that we were suggesting to our customers provided real benefits because the manufacturing setup that we designed for them produced the product diligently and in a very optimized way,” he explains.
He had noticed pharmaceuticals weren’t using simulation the way other industries he worked with were. There wasn’t much investment in computer model and simulation (CM&S) in the pharma industry.
“They had lots of appreciation for in silico methods, but the value benefit they were deriving from the simulations wasn’t great because they were stopping at certain design stages,” David says. “They couldn’t create the entire value chain.”
Additionally, the U.S. Food and Drug Administration (FDA) and other regulatory agencies weren’t mandating or even allowing pharmaceutical companies use simulation to justify their decisions during the regulatory approval process; hence pharmaceutical companies weren’t interested in navigating the CM&S journey. But that has changed.
Being First to Market with Simulation
The first to market advantage is even more significant when the first generic product makes it to market one month or more before subsequent market entrants. In these cases, they can enjoy a 90% market share advantage in drug development. “If you miss this chance, you will miss your revenue,” David says. Simulation can help pharmaceutical companies beat the competition by reducing product iterations at common good manufacturing practice (CGMP) compliant manufacturing plants.
From a regulatory aspect, simulation can provide necessary digital evidence to regulatory bodies justifying why a pharmaceutical company may decide to create their product the way they have and the interlinkages between CMAs (critical material attributes), CPP (critical process parameters) and CQAs. “But the FDA wants so many facts about your product, and you cannot justify it using whatever means you want,” David says. “You need to explain anomalies.”
Simulating Solutions at Dr. Reddy’s
When David started at Dr. Reddy’s, there were some challenges that he quickly solved with simulation, such as different particle sizes distribution in mixing tanks. Lower particle size increases dosage uniformity and the solubility of the drug compound. “They had spent four to six months and incurred a huge cost trying to solve this problem,” David says. “We simulated it and within a couple of weeks we had a single-phase solution and it worked.”
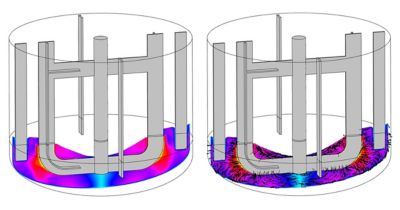
In drug manufacturing, companies must follow CGMPs set up by the FDA and other regulatory agencies. Following CGMPs requires vast amounts of documentation, so changing one aspect of a setup can take weeks, resulting in loss of revenue and time. This is especially evident in different mixing profiles, or the way ingredients are added to the mixing tank.
“If you don't know what the mixing profiles are, you will keep experimenting in the manufacturing setup,” explains David. “One batch can take two weeks. If you guessed wrong, that’s a lot of lost revenue and time.” But by building in simulation to the setup and manufacturing processes, you accumulate a lot of knowledge about your process, resulting in less iterations so the scaling up process is faster, cheaper, and much easier.
The Future of Pharma
Even though competition is strong, opportunities haven’t increased much in the pharmaceutical industry. It is imperative that firms get to market first. Because of this, David has witnessed an increasing interest across the pharmaceutical industry in modeling and simulation. “For the last four and five years, people are at least aware of the benefits simulation can provide,” David explains.
But it is more common for medical devices or delivery device manufacturing to use simulation versus drug manufacturing. “They basically design their devices to understand the drug and device combination and to solve some of the multiphysics problems,” says David.
In India, where David is based, there are a handful of other companies using simulation for drug manufacturing. Because Dr. Reddy’s is an early adopter of modeling and simulation technology, they have been reaping the benefits for roughly four years. The rest of the industry hasn’t seen the benefits the way Dr. Reddy’s has, so they aren’t investing in it as heavily yet. “I would say Dr. Reddy’s has really been on the frontier of this technology,” David says.